Table of Contents
Introduction
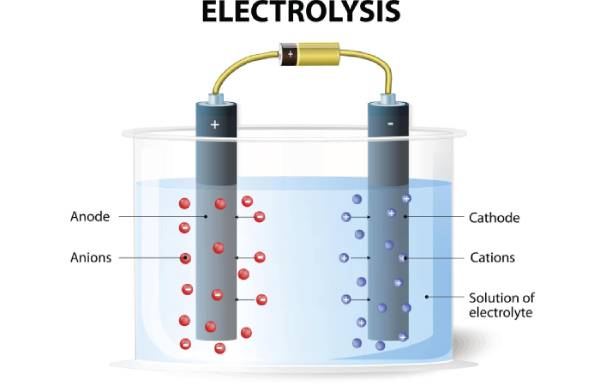
An anode is an oxidation-occurring electrode. It is where the electrons exit from the system, as oxidation is the loss of electrons. Furthermore, an anode is how the electric current flows into polarized electrical devices.
Anodes are available in several devices like batteries, fuel cells, and analytical cells in electrochemistry. They also originate in electronic devices like vacuum tubes and LEDs.
Key Properties of Anode:
Depending on the context in which they are used, the properties of anodes vary. Below are some common properties:
- Typical materials of anodes include metals like zinc, aluminum, and graphite. These metals are typically good conductors of electricity as they allow the flow of electrons.
- As it is crucial to maintain the stability and integrity of the anode periodically, they must resist corrosion. This is particularly relevant in scenarios where anodes are subjected to corrosive conditions.
- Anodes are ideal because of their ability to undergo oxidation reactions in electrochemical systems. They should effortlessly give up electrons during the electrochemical process.
- In a circuit, anodes are the source of electrons.
- They are the source of converting chemical energy into electrical energy.
Types of Anode:
- Sacrificial anodes: The design of these anodes intends to be corrosive to protect another metal from corrosion.
- Galvanic anodes: The uses of this type extend to galvanic cells to produce electricity.
- Inert anodes are usually made of platinum or graphite and are unusable during the electrochemical reaction.
Applications of Anode:
Depending on the context, anodes have various applications, primarily in electrochemical processes. Here are some typical applications of anodes:
- Batteries: Anode oxidizes and releases electrons upon the discharging of the battery. In a battery, it is in the location of the negative terminal.
- Fuel cells: In a fuel cell, fuel oxidation occurs at the anode, releasing electrons through the circuit to the cathode, which combines with oxygen to produce water.
- Electrolytic cells: Here, oxidation of the electrolyte occurs at the anode. Therefore, it releases electrons traversing the circuit to reach the cathode and reduce the cathode material.
- Vacuum tubes: In a vacuum tube, they are the positively charged electrodes drawing electrons discharged from the cathode. Hence, it produces the current with the flow of electrons amid the cathode and the anode.
- LEDs: To draw electrons from the semiconductor, they function as a positively charged electrode in LEDs. Subsequently, they recombine with holes within the semiconductor, which results in the emission of light energy.
Conclusion:
In conclusion, anodes are critical components in various electrochemical processes and play key roles in batteries, electroplating, corrosion protection, electrolysis, and other applications.
Additionally, anodes facilitate the flow of electrons, supporting the production of electrical energy or the progression of specific chemical reactions. Their properties, including conductivity, corrosion resistance, and electrochemical activity, improve performance in various contexts.
They play a diverse and vital role in modern technology and industry, from powering electronic devices to protecting structures from corrosion.